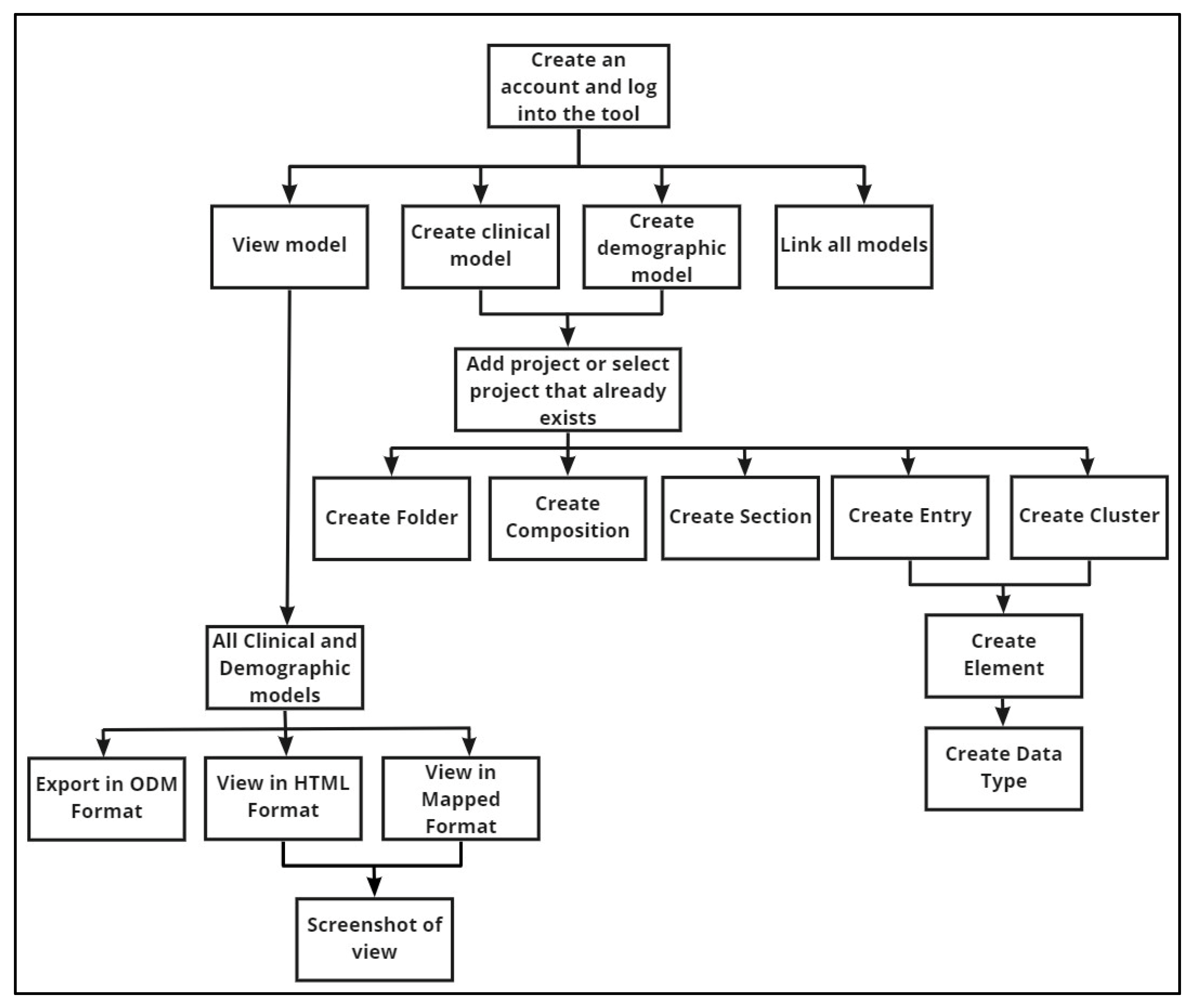
Applied Sciences | Free Full-Text | How Can a Clinical Data Modelling Tool Be Used to Represent Data Items of Relevance to Paediatric Clinical Trials? Learning from the Conect4children (c4c) Consortium

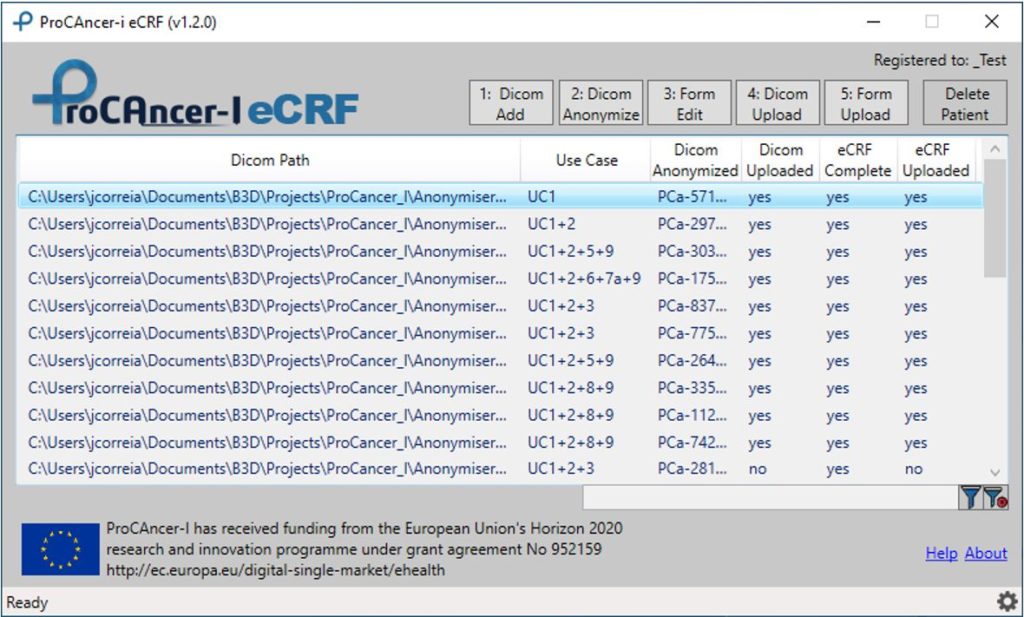
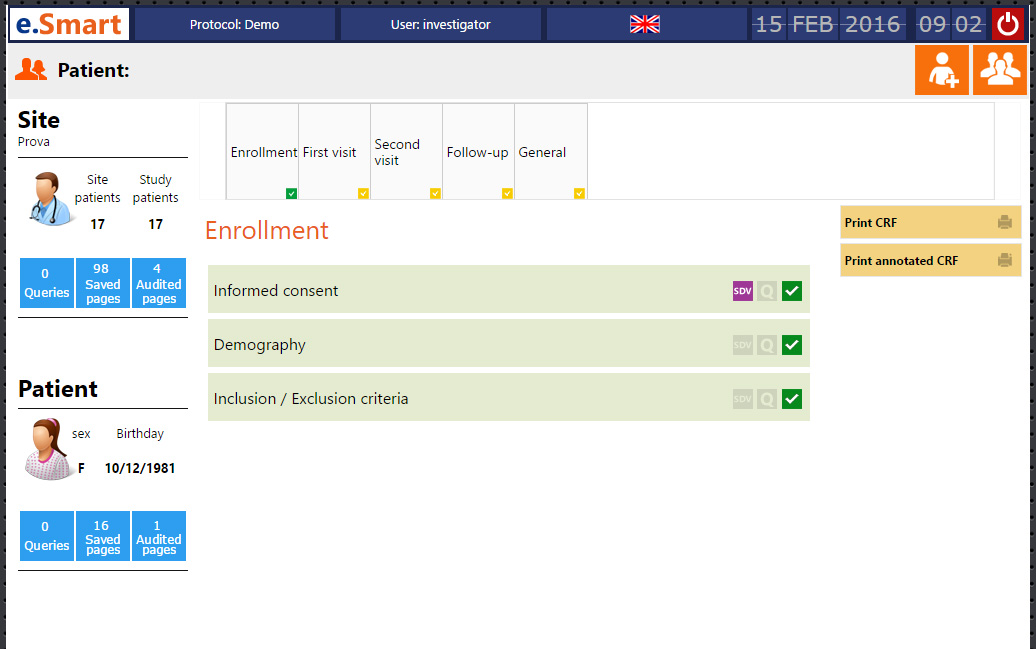
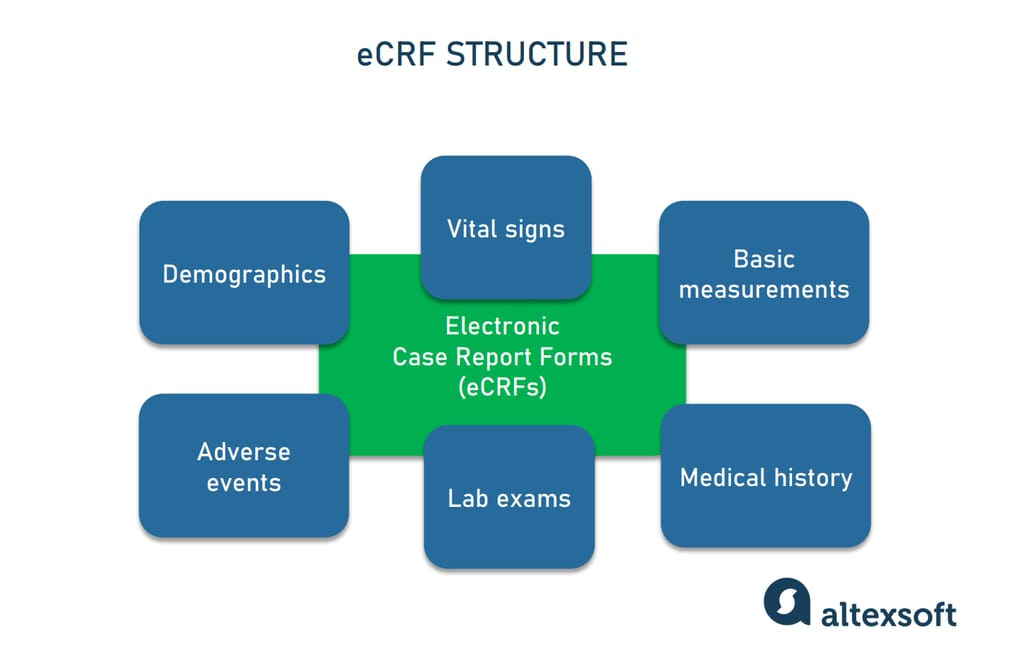
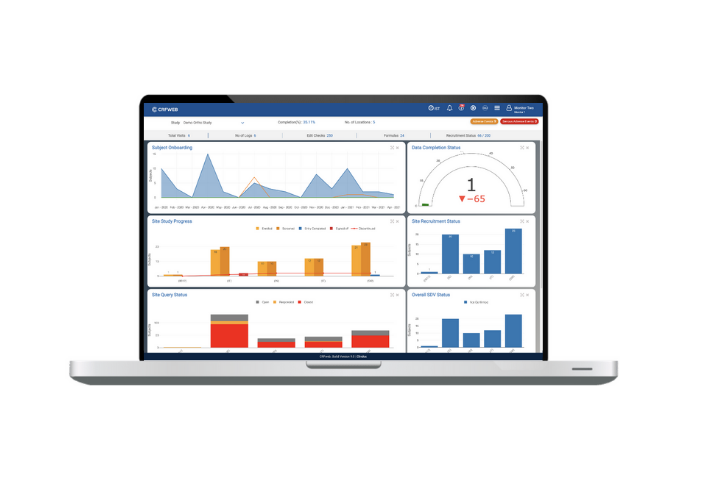
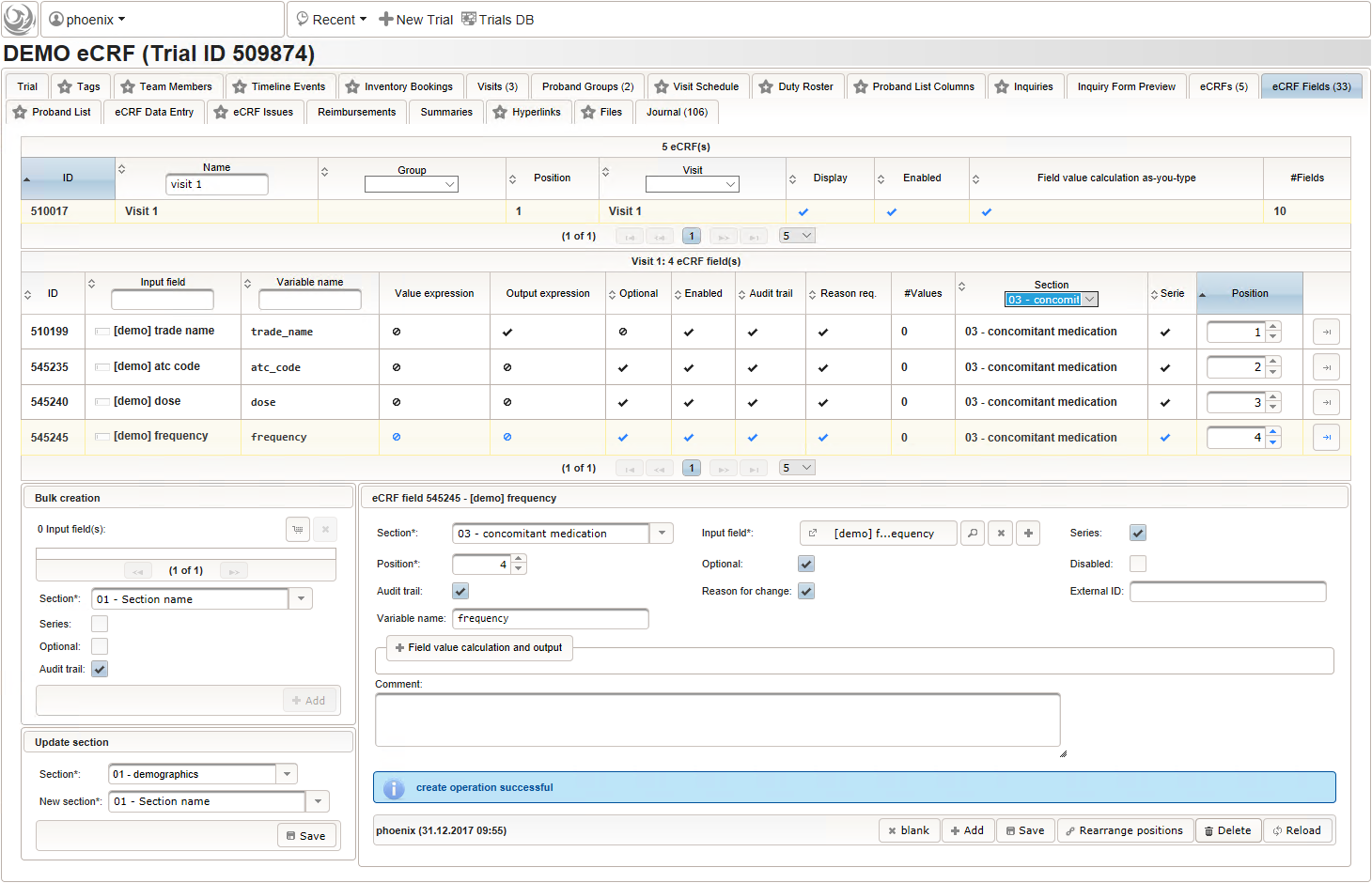
A schematic of data flow in a typical clinical study. eCRF, electronic... | Download Scientific Diagram


%20for%20Medical%20Device%20Studies%20-%20new.png)










.png)